Abstract
Background: Chronic myelomonocytic leukemia (CMML) is the most common myelodysplastic/myeloproliferative (MDS/MPN) overlap syndrome. Patient (pts) exhibit features of myeloproliferation, such as leukocytosis and splenomegaly, as well as cytopenias resulting from ineffective hematopoiesis. CMML patients comprise an elderly and frail population and overall survival is less than 3 years. Treatment options such as hypomethylating agents (HMAs) have limited efficacy and do not alter mutational burden. More than 50% of pts with CMML have somatic mutations that activate the RAS/MAPK signaling pathway.
Cobimetinib is a highly selective oral small molecule inhibitor of mitogen-activated protein kinase kinase 1 (MEK1) and MEK2. MEK1 and MEK2 activate extracellular signal-regulated kinase 1 and 2 (ERK1/2) through phosphorylation of serine/threonine and tyrosine residues, resulting in activation of RAS/MAPK transcriptional signaling. Cobimetinib is approved for use with vemurafenib in the U.S. and Europe for advanced melanomas harboring BRAF mutations. MEK inhibitors have demonstrated efficacy in preclinical models of RAS activated CMML, but their clinical efficacy in CMML is unknown. We designed a phase 2 trial to test the efficacy of cobimetinib monotherapy in CMML pts preselected for RAS/MAPK activating mutations.
Methods: This is an open label, prospective, investigator-initiated study of single agent cobimetinib in CMML patients with RAS pathway mutations (NCT04409639). Two cohorts of pts (newly diagnosed/untreated and HMA-exposed) will be accrued using Simon's two-stage design (sample size n=29). Cobimetinib dosing is 60mg daily on Days 1-21 of a 28-day cycle. Response is assessed with bone marrow biopsy every 3 months. The primary endpoint is overall response rate according to 2015 MDS/MPN-IWG criteria. Secondary endpoints include frequency of adverse events, proportion of pts achieving CR+PR and 36-month progression-free and overall survival. Safety monitoring includes cardiac ultrasound and ophthalmologic exams at regular intervals. Blood and bone marrow samples are collected longitudinally for correlative studies. The trial is currently enrolling at the University of Utah Huntsman Cancer Institute (HCI) and multisite opening is imminent.
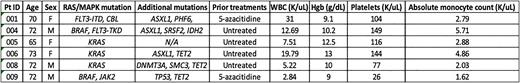
Results: Between January 2021 and July 2022, 9 pts were screened, and 6 pts were enrolled on trial at HCI. The median age of pts enrolled on study was 72 years. Three of six pts were female (50%). Four pts had received no prior treatment for CMML, and two pts had been previously exposed to hypomethylating agents. Three pts had CMML-0 and 3 pts had CMML-2 at screening. One pt required hydroxyurea (HU) for cytoreduction prior to starting cobimetinib. Baseline CPSS-Mol risk was as follows: Low (2 pts), Int-1 (1 pt), Int-2 (2 pts) and High (1 pt). Baseline blood count parameters at screening are listed in Table 1. Pts had mutations in KRAS (50%), BRAF (33%), FLT3 (33%), CBL (17%) and JAK2 (17%). Five pts had a normal karyotype, and one had del (13q). Additional somatic mutations are listed in Table 1. Rapid reductions in absolute monocyte count and improvements in Hgb and platelet count were observed in 4/5 evaluable pts after initiation of cobimetinib therapy, and the pt requiring HU for cytoreduction maintained normal blood counts after HU discontinuation. Responses were evaluable for at least one timepoint in 4/6 pts and encompassed progressive disease (1 pt), partial marrow response with erythroid response (1 pt), spleen response (1 pt) and stable disease (1 pt). Two pts discontinued cobimetinib. Reasons for treatment discontinuation were progression to AML (1 pt and) Grade 3 mucositis (1 pt). 2 pts required dose reduction to cobimetinib 40mg daily for mucositis (1 pt) and diarrhea/rash (1 pt). The most common treatment related AE was diarrhea (83%), all cases of which were Grade 1 or 2 and easily managed with the addition of loperamide. Other treatment related AEs observed in at least 2 pts included maculopapular rash, edema, and fatigue. No deaths were observed on study and all pts enrolled remain alive at time of data cutoff.
Conclusions: Oral cobimetinib showed single agent activity (ORR 50%) and safety in a small number of CMML patients. Accrual for NCT04409639 is ongoing and multisite opening is in progress. Correlative studies, including measurement of circulating inflammatory cytokine levels and mutant allele burden are ongoing and will be presented.
Disclosures
Patel:Incyte: Research Funding; Stemline: Research Funding; Genentech/Roche: Research Funding. Osman:Syros Pharmaceuticals: Research Funding; Karyopharm Therapeutics: Research Funding. Tantravahi:Karyopharm Therapeutics Inc.,: Research Funding; Karyopharm Therapeutics Inc., Novartis, AbbVie, Incyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Deininger:Pfizer Inc: Consultancy, Honoraria, Research Funding; Galena Biopharma: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Honoraria, Research Funding; Incyte: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Blueprint Medicines Corporation: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal